1436
Views & Citations436
Likes & Shares
Background: Candesartan Cilexetil is widely
prescribed antihypertensive. The drug is unfortunately available in the market
only as tablets which are not suitable for children and patients on nasogastric
feeding.
Objectives: The study aimed to formulate and
evaluate an appropriate extemporaneous oral suspension of candesartan
Cilexetil.
Methods: Six oral suspension formulations
of the drug were prepared from the 16 mg tablets of the innovation brand
(Atacand®) of the drug. All formulations were prepared as “flocculated
suspensions with a structured vehicle as a final product” with differences in
the speed of mixing applied.
Results: Formulations with high-speed
mixing were more viscous and more difficult to be poured than those prepared
using low-speed mixing. Of the later, one formulation with increased amounts of
flocculating agent, viscosity enhancer and wetting agents showed proper
physical stability, flocculation and a shelf-life of more than 1 year.
Conclusion: A stable oral suspension of
candesartan Cilexetil extemporaneously prepared from the drug tablets which can
be easily prepared by pharmacists using available constituents, is invented in
this study.
Keywords: Candesartan, Extemporaneous, Oral
suspension, Formulation, Evaluation
INTRODUCTION
Pharmacists
are the health professionals who are trained to perform extemporaneous
compounding and it is a required competency of practice for registered
pharmacists in many countries [1].
Extemporaneous
compounding is the preparation of a therapeutic product for an individual
patient in response to an identified need. It is a practical way to have medicines
supplied when there is no other option. For example, compounding may be useful
for patients with dysphagia who are unable to swallow solid medications whole,
when an appropriate dose or dosage form is not commercially available, when
patients require an individualized dose, or when medicines must be delivered
via nasogastric or gastrostomy tubes.
It should
take place in community and hospital pharmacies. There are usually specialist
compounding pharmacies in major towns and cities, but any pharmacy may
undertake compounding as long as they have appropriate facilities according to
country-based legislation [2].
For
compounding, the active ingredient may be derived from commercially available
medications or the pure chemical. Sometimes compounding is as simple as mixing
a crushed tablet or the contents of a capsule in water to form a solution or
suspension. However, this may not be suitable and depends on the solubility of
the active ingredient. For example, insoluble tablet excipients can lead to blockages
in enteral feeding tubes. In the majority of compounded products, additional
non-active components (excipients) are
included to ensure the active ingredient
dissolves or remains suspended, or to adjust palatability or viscosity [3].
Risks in
off-label compounding include using incorrect
formulae and calculations, selecting
incorrect ingredients, using incorrect quantities and producing unstable
products [1]. In community compounding, if a preparation error occurs, it would
only affect a limited number of patients. . Conversely, when pharmacy compounding
is done at a large scale e.g. in
hospitals
the error could potentially affect a large population of patients [4].
Candesartan
Cilexetil [1H-Benzimidazole-7-carboxylic acid, 2-ethoxy-1-[[2¢-(1H-tetrazol-5-yl)
[1, 1¢-biphenyl]-4-yl] methyl]-, 1[[cyclohexyloxyy) carbonyl] oxy] ethyl ester
[5] is an oral drug belonging to the category angiotensin II receptor blockers
(ARBs). It is prescribed for hypertension (adults and children) and also for
congestive heart failure (adults). The dose for children (1-6 years) is 0.2
mg/kg a day or divided every 12 h, (6-17 years) 4-8 mg day. The drug is
available commercially as tablets (4, 8, 16 and 32 mg) [6,7]. The drug is
practically insoluble in water [8]. It has pka value of 6.0 and its partition
coefficient (Octanol/aqueous) at pH 1.1, 6.9 & 8.9 is >1000 indicating
high hydrophobicity character [9].
Physicians
in Yemen sometimes prescribe candesartan Cilexetil for patients who cannot
swallow the tablet form including children or patients on nasogastric feeding.
Unfortunately, the drug is only available only as tablets. Therefore, for those
patients, they recommend to crush the tablet and mix it with milk or juice and
give it to the patient peroral. This is absolutely a non-evidence-based
practice since no studies have revealed its validation. Hence, the need for
valid extemporaneous liquid preparation of the drug becomes mandatory.
METHODS
Materials
& instrumentation
Reference standard of candesartan Cilexetil
was a gift from Yemeni- Egyptian Pharma Co., Sana`a, Yemen. Atacand®
(Candesartan Cilexetil 16 mg tablets; Astra-Zeneca, Switzerland) was a gift
from In-patient pharmacy at Al-Thawra Public hospital. UV spectrophotometer
(Shimadzu, UV-1800, Japan), high speed Lab Disperser & mixer (AD500S-P,
Mxbaoheng, China).
Assay of Candesartan Cilexetil in Atacand® tablets
The assay
was conducted as described by Ravisankar P et al. [10]. A stock solution of the
standard reference of the drug was prepared in methanol (100 µg/ml). Serial
dilution of the stock solution was performed to yield 6 dilute standard
solutions of concentrations of 10-50 µg/ml. The absorbances of the solutions
were measured at 258 nm. The standard calibration curve was constructed and its
linearity equation was determined. Twenty tablets of Atacand® 16 mg
(theoretically contain 320 mg of Candesartan Cilexetil) were ground to powder
and sieved through mesh No. 60. A quantity of the sieved powder equivalent to
16 mg of the drug was taken and dissolved in methanol up to 100 ml. Dilution of
the resultant solution was made to provide a dilute solution with a theoretical
concentration (Ct) of 40 µg/ml whose UV absorbance was measured at 258 nm. The
test was conducted in a triplicate manner. The UV absorbance was introduced
into the calibration linearity equation to calculate practical concentration of
the drug (Cp). Drug content was then calculated as follows:
Drug content % = 100 x Cp / Ct
Formulation of oral
suspensions from Atacand® tablets
Six suspension formulations (Table 1) were prepared using the
method of “flocculated suspension with structural vehicle” [11]. Atacand® 16 mg
tablets were used as source of active ingredients. Twenty tablets were ground
to powder and sieved through mesh No. 60. The powder was then levigated with
glycerin and polysorbate 20. Sodium chloride (flocculating agent) was dissolved
in few amount of water and then was added to the levigated mass and triturated.
Then, the structured vehicle was prepared as a solution of methylcellulose in
an amount of water representing two-third of the final suspension volume. All
other ingredients was dissolved in the structured vehicle. The resultant
solution was added into the flocculating-added levigated mass with thoroughly
stirring by the mixer for 15 min. The produced suspension was transferred to a
volumetric cylinder and the volume was completed to the desired volume with water.
Evaluation of oral suspension formulation
Drug content
To 1 ml of the suspension (theoretically
contained 16 mg candesartan Cilexetil), 7 ml of water was added and the mixture
was centrifuged at 2000 rpm for 12 min. The supernatant was discarded and 10 ml
of methanol was added to the residue and the resultant was then filtered. The
volume of filtrate was completed to 16 ml with methanol. 1 ml of this solution
was then diluted to 40 ml with methanol producing a theoretical concentration
(Ct) of 40 µg/ml. The UV absorbance at 258 nm of the solution was measured. The
test was carried out in triplicate pattern. The drug content was then
determined as described earlier in assay of the drug in Atacand tablet [10].
Physicochemical evaluation
The particle size, sedimentation rate, pH,
sedimentation volume, degree of flocculation and viscosity of the formulations
were measured.
Particle size and sedimentation rate
They were measured as described in
literature [11, 12] by sedimentation method by standing for 24 h using Stock`s
Equation as follows:
d = 104. [(S.18.Y) /
((Ps –Po).g)] 1/2
where (d) was the particle size as
diameter of dispersed particles (µm), (S) was sedimentation rate (cm/s), (Y)
was viscosity of the formulation (dyne.cm-2s), (Ps) was
the density of dispersed particles (g.cm-3), (Po) was the
density of formulation vehicle (g.cm-3) and (g) was the acceleration
due to gravity (980.7 cm.s-2).
• Sedimentation
volume [11] was calculated after standing for 24 h as follows:
F = Vs/Vo
where (Vs) was the sediment volume after 24 h
standing and (Vo) was the original suspension volume.
• Degree of flocculation [11]
It was measured by comparing the
sedimentation volume in the flocculated formulation to that of its
corresponding (sodium chloride free) suspension as follows:
β = F∞ /F
where (F) was the sedimentation
volume of the flocculated suspension and the sedimentation volume of the
suspension when deflocculated (F∞). As (β) increases (> 1) the
volume of sediment in the flocculated system is greater than that in the
deflocculated state which confirms the flocculation of the system.
•Viscosity of the suspension [13]
It was measured using Ostwald tube using H2O
as reference standard (H2O) which has a viscosity of (0.01 dyne cm-2
s).
Viscosity of the suspension was calculated as
follows
Y s = ts
x Y w / tw
where (Y s) and (Y w) were viscosity in (dyne.cm-2s)
of the suspensions and water, respectively, and (ts) and (tw)
were flow times of the suspension and water, respectively.
Accelerated stability study [13]
The suspension formulation with appropriate
drug content and physicochemical properties was selected for stability study in
order to determine its shelf life.
A sample of 50 ml of this
formulation was incubated at three different conditions 37, 50 and 75°C for 6 weeks. At intervals of 2,
4 and 6 weeks, each formulation was tested for its drug content. The results of
drug content were used to predict the shelf-life using Arrhenius equation. The
order of degradation reaction was determined by fitting data of drug content
vs. time to zero, first and second-order models at each storage temperature.
The model that showed higher correlation coefficient was the order of
degradation to which data fitted at a given temperature and the rate constant
of degradation (k) of that order was then determined. Arrhenius plot was
constructed with ln k (at y axis) versus I/T (at x axis); where T was the
temperature of storage in Kelvin. The regression equation of the plot was then
determined where the slope and intercept was used to calculate the expected
degradation rate constant at 25°C (K25) as follows
ln K25 =ln A – [(Ea/R). (1/T25)
where (ln A) was the intercept;
(Ea/R) was the slope of the plot.
The expected shelf-life (t90)
was calculated as follows
t90 = (0.1 * Q0)/K25
where Q0 was then drug content %
at the beginning of the stability study.
RESULTS
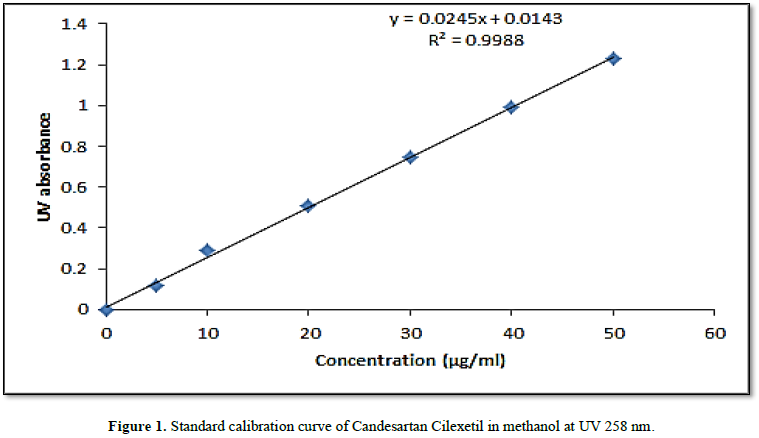
Standard calibration curve
A linear curve was obtained for
concentrations (10-50 µg/ml) of candesartan Cilexetil standard in methanol. The
linearity coefficient was 0.9988 and the curve equation was (y = 0.0245x +
0.0143).
Drug content in Atacand® 16 mg tablets
The average ± SD of drug content %
was 100.15 % ± 0.05 (CV was 0.05%; C.I. 95 % was (100.093 – 100.207 %).
Evaluation of suspension formulation
Drug content
The drug content of the drug in
all formulations, as shown in Table 2,
was within the range of 98.7–101% (95 % C.I. 92.422 -106.578 %). CV% (the
coefficient of variation) of all measurements, within each formulation, were
<5% indicating optimum precision.
Physicochemical properties
As shown in Table 1, there were two categories of formulations (Fa) with lower
mixer speed and (Fb) with higher mixer speed) and each category was divided
into 3 subcategories according to the amount of wetting agents, flocculating
agent and suspending agent where the formulation with the numbers 1, 2 and 3
denoting the smallest, intermediate and largest amounts of such
Table 3
demonstrates the results of physicochemical properties which showed significant
smaller particle size, higher degree of flocculation and lower sedimentation
rate of (Fb) formulations compared to (Fa) formulations. On the other hand,
there were no significant differences between (Fa) and (Fb) formulations
regarding sedimentation volume and pH. It was found that (Fb) formulations had
very large viscosity that made the suspension very difficult to be poured and
hence were excluded from further investigations.
Accelerated stability study
The formulation (Fa3) which showed proper
drug content, moderate viscosity, proper physical stability and flocculation
was introduced into an accelerated stability study. The order of degradation of
the drug at all the three storage temperatures fitted to first-order model.
Data obtained from Arrhenius plot was demonstrated in Table 4. The shelf-life of the drug in that formulation was 1.27
years.
DISCUSSION
Candesartan Cilexetil is yet available in the
market as commercial oral suspension. Instead, the innovator (Astra Zeneca)
recommends a method to prepare oral suspension from the available tablets [8].
The method requires the use of a specific vehicle products e.g. Ora plus (Perrigo,
Australia) which are unavailable in the Yemeni drug market.
The vehicle is composed of many
ingredients including: microcrystalline cellulose, carboxy methylcellulose,
xanthan gum, carrageenan, calcium sulphate, trisodium phosphate, citric acid,
dimethicone antifoam emulsion, methyl paraben and potassium sorbate [14]. Most
of these ingredients are expensive and unavailable in Yemen.
Crushing of Candesartan Cilexetil tablet then
mixing it with fluid has been recommended by some physicians in Yemen for
patients who cannot swallow tablets such as children. This practice may be
associated with great risks to patients’ due potential errors in formulation
and stability of the drug. Therefore, the present study aimed to offer another
option to physicians, patients by formulating a stable extemporaneous oral
liquid of the drug which can be simply prepared by pharmacist.
Therefore, this study was
undertaken in order to provide an oral suspension formulation with simple
formulation from available ingredients.
The
drug was formulated as an aqueous suspension owing to the numerous advantages
of this liquid dosage form including the non-necessity of dissolving the drug,
which is insoluble in water, the ability of suspension to mask unpleasant taste
of the drug and the enhanced chemical stability of drugs when formulated as a
dispersion system [13].
Prior to formulation, it was
necessary to confirm the drug content in the tablets. This was carried out
using the simple technique of UV spectrophotometry. A standard calibration
curve was constructed and revealed high linearity (Figure 1) and the drug content in tablets complied the USP
specifications (90-100%) [5].
The formulations were prepared
from the (Atacand® 16 mg tablets) which is a brand widely available in the
market. This is an advantageous point as pharmacists can prepare the drug as
oral suspension whenever required with no need to import the raw material of
the drug which is considered uneconomic due to limited request of the oral
suspension of the drug.
As shown in Table 1, six suspension formulations were designed based on
preformulation data of the drug and the required excipients. All formulations
were prepared as “flocculated suspension in structured vehicle as final
product”. This system is well known with improved physical stability of the
product as it prevents sediment cake formation (flocculated system), enhances
the viscosity and reduces sedimentation rate (structured vehicle) [11,13].
The differences among those formulations were
in the in the speed of mixer applied (low speed 1000 rpm or high speed 6000
rpm), the amount of flocculating agent (sodium chloride), viscosity enhancer
and structured vehicle (methylcellulose solution), wetting agents including the
surfactant (polysorbate 20) and glycerin. Other ingredients included were of
the same type and quantity in all formulations. They included sodium dihydrogen
phosphate and dibasic sodium phosphate as a buffer pH 6.8, sodium benzoate as
preservative, sodium sulphite as antioxidant, ethyl maltol as caramel flavor,
caramel E 150 as coloring agent and saccharin sodium as sweetener [11,13].
Evaluation of the prepared formulations
reveled optimum drug content in all formulations and the precision of
measurements was confirmed with low CV% (Table
2). With respect to physicochemical properties, as shown in Table 3, the formulations prepared at
high-speed mixing (Fb1, Fb2 & Fb3) though were of better physical stability
characters with lower particle sizes and sedimentation rates, they showed very
high viscosity most probably due to thixotropic phenomenon [11,13].
Accordingly, they were very difficult to be poured and as a result they were
excluded from further investigations. The physicochemical properties of (Fa)
formulations were within standard suspension specifications. The particles size
was in the range of 11.2-18.3 µm compared to standard coarse suspension of
10-50 µm [11], the formulations viscosity was accepted (1.01-3.1 dyne cm-2
s) as it was more than that of water (1.01-3.1 dyne cm-2 s) [11] and
the degree of flocculation was >1 within the range of (1.2-5.1) which
confirmed the appropriate flocculation of the particles in the prepared
suspensions [11].
Among the low-speed mixing formulations, Fa3
which contained an increased amount of flocculating agent, wetting agents and
viscosity enhancer showed more appropriate characters in terms of higher
viscosity, lower particle size, higher sedimentation volume, higher degree of
flocculation and lower sedimentation rate than the other two formulations (Fa1
and Fa2).
In the last step of evaluation,
the selected Fa3 formulation demonstrated considerably long shelf life of more
than 1 year (Table 4) compared to
only 100 days reported with the use of oral-plus as vehicle [7].
CONCLUSION
The suspension formulation of Candesartan
Cilexetil (Fa3) prepared in this study from the drug tablets using simple
method and available constituents is a stable oral suspension of the drug that
can be extemporaneously prepared by pharmacists whenever an oral liquid
preparation of the drug is required.
CONFLICT OF
INTERESTS
The authors declare that there is no conflict
of interests regarding the publication of this article.
AUTHOR CONTRIBUTION
The correspondent author conceived the idea
and developed the theory of the presented work. All authors participated in
conducting experiments, performing the calculations, discussing the results and
contributing in the last manuscript.
1.
Kairuz TE, Gargiulo D, Bunt C, Garg S
(2007) Quality, safety and efficacy in the ‘Off-Label’ use of Medicines. Curr
Drug Saf 2: 89-95.
2.
James FR (2017) Extemporaneously
compounded medicines. Aust Prescr 40: 5-8.Jain A (2006) Waste management in Asian countries. UN Environment.
3.
British Association for Parenteral and Enteral
Nutrition (BAPEN) and British Pharmaceutical Nutrition Group (2004) Administering
drugs via enteral feeding tubes: A practical guide 2019.
4.
Gudeman JJ, Jozwiakowski M, Chollet J,
Randell M (2013) Potential risks of pharmacy compounding. Drugs R D 13: 1-8.
5.
United States Pharmacopeia and National
Formulary (2010) Rockville, MD: United States Pharmacopeial Convention (USP
33-NF 28).
6.
Medscape (2019) Candesartan Rx.
Accessed on 15th December, 2019. Available online a: https://reference.medscape.com/drug/atacand-candesartan-342314.
7.
Atacand (2016) Product monograph. Accessed
on 25th December, 2019. Available online at:
https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/ATACAND%20-%20Product-Monograph_001.pdf
8.
Atacand (2016) Highlights of prescribing
information. Accessed on 16th December, 2019. Available online at
https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020838s039lbl.pdf
9.
Cagigal E, Gonzalez L, Alonso R.M, Jiménez
RM (2001) pKa determination of angiotensin II receptor antagonistic (ARA II) by
spectrofluorimetry. J Pharm Biomed Anal 26: 477-486.
10.
Ravisankar P, Vidya Sree V, Manjusha K, Naga
Mounika Y, Srinivasa Babu P (2016) Validated UV spectrophotometric method for
quantitative determination of Candesartan Cilexetil in bulk and pharmaceutical
dosage form. Der Pharmacia Lettre 8: 252-257.
11.
Felton L (2013) Remington essential of
pharmaceutics, Pharmaceutical Press. The Royal Pharmaceutical Society, UK, pp:
371-378.
12.
Shid R L, Dhole SN, Nilesh K, Shid SL
(2013) Formulation and evaluation of nanosuspension for drug delivery of Simvastatin.
Int J Pharm Sci 22: 98-106.
13.
Aulton ME (2012) Pharmaceutics: The
science of dosage form design. Churchill Livingstone, UK.
14.
Preggio Australia (2019) Ora-Plus.
Accessed on 7th March 2020. Available online at:
https://www.perrigo.com.au/product/ethical-care/ora-plus/
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Alcoholism Clinical Research
- Journal of Spine Diseases
- International Journal of AIDS (ISSN: 2644-3023)